Single Cell Mechanobiology
Many factors are reported to influence the mechanical state of cells. These include interactions with the extracellular environment, differentiation status, the onset of disease states, as well as intracellular factors, for example, the cytoskeleton. The contributions made by AFM to the field of cell biomechanics has been invaluable. The technique can be used to measure characteristic mechanical properties of cells, e.g., elasticity, viscosity, plasticity and adherence forces. In addition, AFM can also be used to elucidate how the mechanical attributes of the cell are related to the underlying structure and function of the cell such as the cytoskeleton and focal adhesion complexes. AFM combined with fluorescence microscopy is a novel method for investigating real-time mechano-signalling in single cells. For example, this approach can be used to investigate cell cycle regulation of both nitric oxide (NO) and calcium (Ca2+) signalling in bone cells. AFM investigations of phase dependent cell elasticity allow correlations between cell stiffness and mechano-responsiveness to be made.
Work is being carried out at present to investigate the effect of loading on the dynamics of the cytoskeleton. Using the combined Confocal-TIRF-AFM system, the aim is to determine the active responsiveness of a single cell to applied stimuli. Real-time cytoskeletal imaging using confocal microscopy is used to uncover the role of actin cytoskeleton remodelling. Using this combined system, we can examine the contribution of the cell cytoskeleton to active force generation. The data obtained from such studies has implications for the fields of tissue- and bioengineering, where prior knowledge of cell mechanobiology is essential for the effective replacement and repair of tissue. Furthermore, studies focused on biomechanics and the biophysical properties of cells have gained increasing importance for the understanding of the onset and progression of disease states, for example cancer, at the cellular level. The representative data below illustrates the varied capabilities of the combined AFM/confocal system. Confocal microscopy was used to generate high quality 2 and 3D images of the cytoskeleton, while, AFM was used to acquire complimentary, high resolution images. This demonstrates cell-specific complexities in this structure and by combining the two techniques, the real-time monitoring of Ca2+ signalling in mechanically stimulated cells can be facilitated.
Figure 1: Confocal microscopy image showing the structure of the actin cytoskeleton in osteoblasts. Actin fibres (red) are the primary stress bearing elements in cells and, as such, determine many mechanical properties. e.g., elasticity. Actin filaments also serve to anchor the cell to extracellular environment via focal adhesions (green). These transmembrane proteins play important roles in mechanical signal transduction.
Figure 2: 3D representation of the actin cytoskeleton of a live osteoblast, cell was transiently transfected with GFP-actin, and the representation was made of z-stack images of the cell using IMARIS software package.
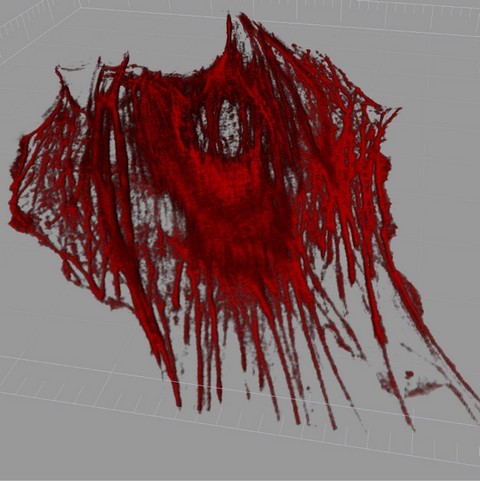
Figure 3: AFM image of a live mesenchymal stem cell, showing geodesic F-actin configurations.
References
AFM imaging and elasticity measurements on living rat liver macrophages, Rotsch, C., Brae, F., Wisse, E., Radmacher, M., Cell Biololy International, 21, 685, (1997).
Stimulation of nitric oxide mechanotransduction in single osteoblasts using atomic force microscopy, McGarry, J. G., Maguire, P., Campbell, V. A., O'Connell, B. C., Prendergast, P. J., and Jarvis, S. P., Journal of Orthopaedic Research, 26, 513-521, (2008).
Bone cells grown on micropatterned surfaces are more sensitive to fluid shear stress, You, J., Reilly, G.C., Zhen, X., Yellowley, C.E., Chen, Q., Donahue, H.J., Jacobs, C.R., Journal of Biological of Chemistry, 276, 13365, (2001).
Atomic force microscopy can be used to mechanically stimulate osteoblasts and evaluate cellular strain distributions, Charras, G.T., Lehenkari, P.P., Horton, M.A., Ultramicroscopy; 86, 85, (2001).
Effect of surface nanoscale topography on elastic modulus of individual osteoblastic cells as determined by atomic force microscopy, Hansen, J.C., Lim. J.Y., Xu, L-C., Siedlecki, C.A., Mauger, D.T.; Donahue H.J., Journal of Biomechanics, 40, 2865, (2007).
Nanomedicine: Elastic clues in cancer detection, Suresh S., Nature Nanotechnology, 2, 748, (2007).
Direct mechanical measurement of geodesic structures in rat mesenchymal stem cells, Maguire, P., Kilpatrick, J. I., Kelly, G., Prendergast, P. J., Campbell, V. A., O'Connell, B. C., and Jarvis, S. P., HFSP Journal, 1, 181-191, (2007). [PDF]
Stimulation of nitric oxide mechanotransduction in single osteoblasts using atomic force microscopy, McGarry, J. G., Maguire, P., Campbell, V. A., O'Connell, B. C., Prendergast, P. J., and Jarvis, S. P., Journal of Orthopaedic Research, 26, 513-521, (2008).
Substrate influence on cell shape and cell mechanics: HepG2 cells spread on positively charged surfaces, Saravia, V., Toca-Herrera, J.L., Microscoscopy Research and Technique, 72, 957, (2009).
Bone Cell Elasticity and Morphology Changes during the Cell Cycle, Kelly, G. M., Kilpatrick, J. I., van Es, M. H., Weafer, P. P., Prendergast, P. J., Jarvis, S. P., Journal of Biomechanics, 44, 8, 1484-1490, (2011).